Analysis of Molecular Processes on Biointerfaces
LOOK AROUND!
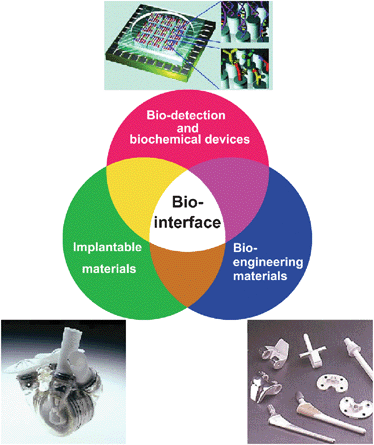
How many interfaces between artificial materials/devices and your body can you find? Ranging from cosmetics, cloths to medical implants, there are a wide variety of materials all around us. When our cells/tissues are in contact with these materials, there will be biophysical and biochemical interactions between cell membrane proteins and the surfaces of materials. These biointerfacial interactions initiate the signalling cascades inside the cells, which determine the subsequent behaviors: adhesion, spreading, migration, proliferation, differentiation, or apoptosis. These cellular behavior collectively will lead to macroscopic responses of our body such as biocompatibility, blood clotting, or even immunological response, etc.
Hayashi Laboratory is trying to understand these biointerfacial interactions through our original techniques for biointerface analyses in order to discuss
1. What are the biophysics and biochemistry behind biocompatibility?
2. What kind of molecular processes occurs between artificial materials and cells or living tissues?
Research Targets
- Ligand-receptor interactions involved in the process of molecular recognition (particularly structure configuration, fluctuation, and dynamics of molecules).
- Identification and structural analysis of protein molecules adsorbed on solid surfaces.
- Water molecules in the vicinity of biocompatible materials and its relevance to biocompatibility.
- Molecular events at cell-material interfaces.
Development of Original Analytical Tools
To observe molecular processes at biointerfaces, we try to develop our original analytical tools. Thus far, we developed the following techniques to investigate biointerfaces:
a) Atomic Force Microscopy for Biointerfacial Imaging
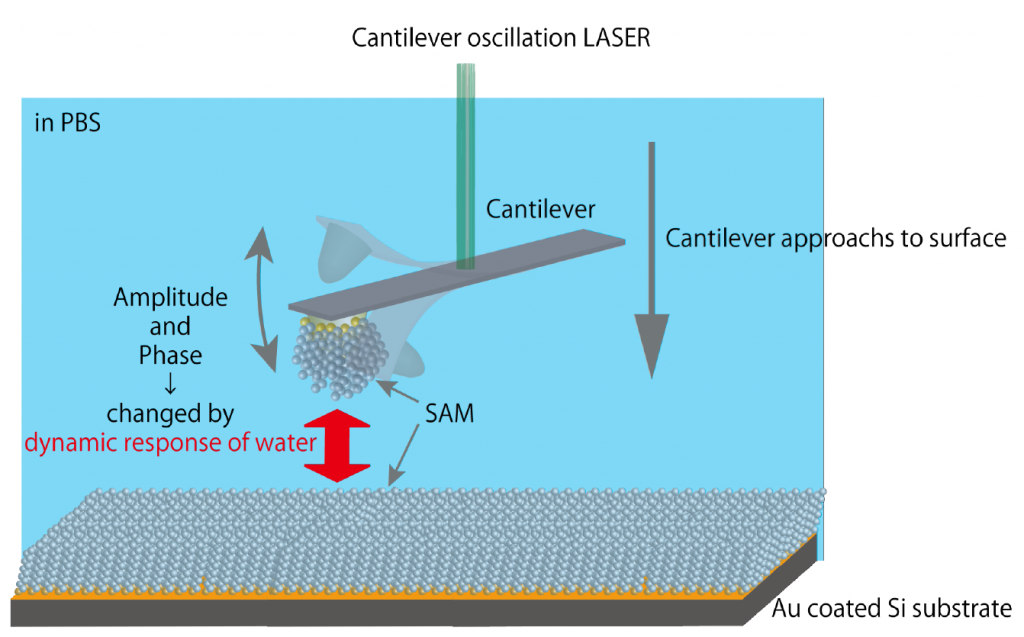
Conventionally, Atomic Force Microscopy (AFM) has been used to image nanostructures of solid materials in vacuum. Now AFM has become a robust tool to visualize biomolecular behaviors at biointerfaces. In addition to conventional piezo-driving of cantilevers, we developed the original photothermal excitation system to oscillate the cantilever with high stability in water, enabling us to explore the cell-material interfaces with an unprecedented resolution.
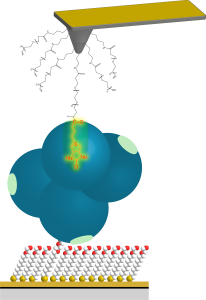
b) Atomic Force Microscopy for Surface Force Analysis and Single-Molecule Force Spectroscopy
AFM also can be used to quantify interaction force between the probe and the surface of materials. Through surface force analysis, we have revealed the behavior of water at biointerfaces. We have clarified that the interfacial water molecules in the vicinity of biointert surfaces serve as steric hindrances (physical barrier) preventing proteins/cells from adsorbing/adhering to the material surface.
Through single-molecular force microscopy, we have quantified molecular adhesion force between cells and materials at a single-molecule level. Molecular interactions at a pico-Newton level (10-12 N) can be revealed by our special force measurements. With this method, we can get the picture of molecular recognition processes of biomolecules.
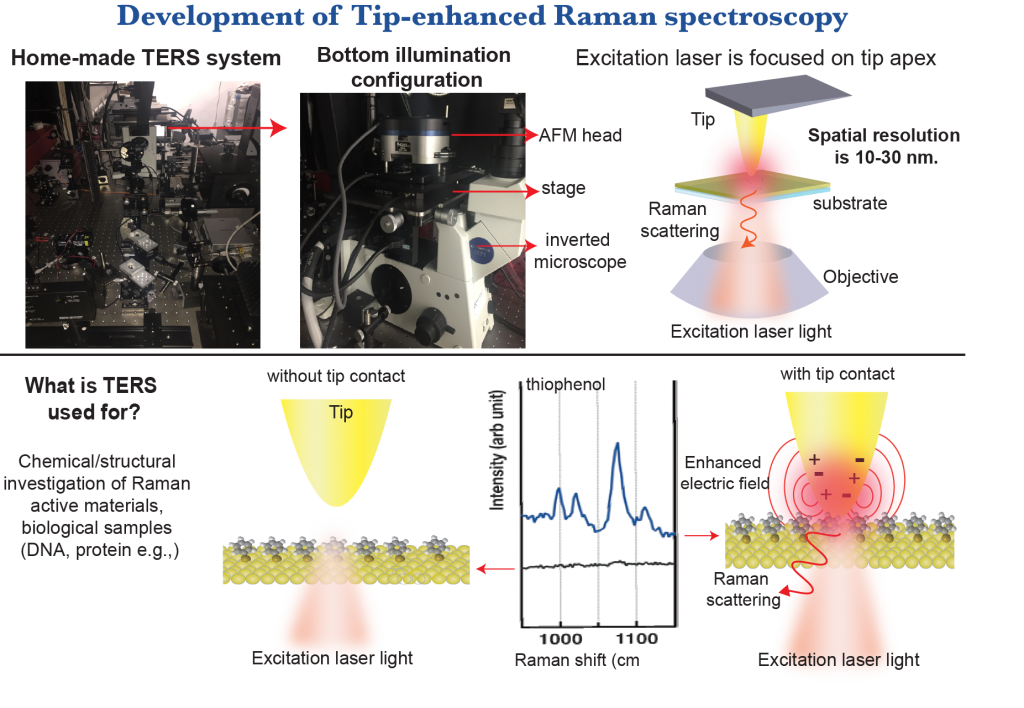
c) Optical Microscopy x Atomic Force Microscope
When AFM is combined with Raman Spectroscopy, the resulting Tip-enhanced Raman Spectroscopy (TERS) enables us to measure local chemical properties of samples with nanoscaled resolution from Raman spectra obtained from the area just under the metalized probe.
Our target samples are not limited to normal inorganic and organic nanomaterials and surfaces. We try to perform diagnostics of single protein molecules, which work as a “bed” for the adhering cells (so-called “ECM”: extracellular matrix)
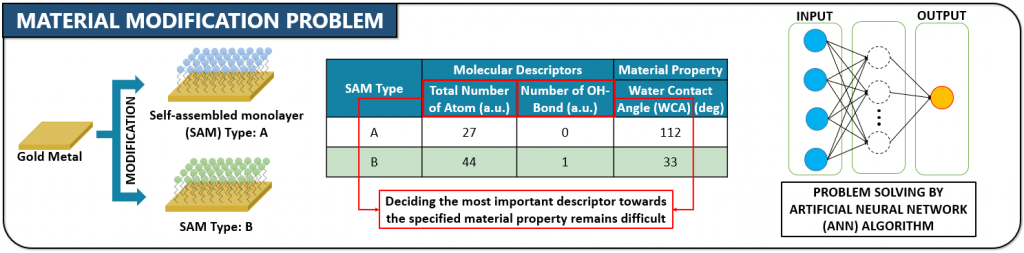
d) Material Informatics For the Optimization of Biomaterial Design
In 2016, we started a new project to design biomaterials using a technique of material informatics.
Keywords for Our Techniques and Materials
Scanning probe microscopy, Atomic force microscopy, Nanophotonics, Single-molecule force measurements of peptides, proteins, ligand-receptor, antibody-antigen molecules